Abstract
Background:
Sickle cell disease (SCD) is a severe monogenic disorder in the world with >300,000 newly born afflicted children per year. Prevalence of this disorder is high in sub-Saharan Africa, Mediterranean basin, Middle East and India. Patients with severe disease phenotype manifested with stroke or other complications may benefit from allogeneic hematopoietic stem cell transplant (SCT). Recent reports in pediatric patients using myeloablative regimen showed excellent outcomes but similar progress in adults has been hampered by higher rates of transplant related mortality (TRM) and infertility.
Methods:
We conducted a study of adult patients at our institution with SCD whom underwent a matched related SCT during the period of 2014-2017. Medical records of eligible patients were retrospectively reviewed and extracted. All patients received hydroxyurea (HU) at maximally tolerated doses with hyper-transfusion 2-3 months prior to SCT. Iron chelation was given for patients with ferritin > 1000 ug/L. The preperative regimen was non-myeloablative (NMA) consisting of alemtuzumab 1 mg/kg divided over 5 days (day -7 to -3) followed by Total Body Irradiation (TBI) 300 cGy on day -1. Stem cells were collected from peripheral blood via apheresis after 5 days of GCSF with target CD34 cells of 10×106/kg. Graft vs. host disease (GVHD) prophylaxis consisted of sirolimus starting from day -2 and continued for one year post SCT with dose modification based on chimerism. Event free survival (EFS) was defined from time to SCT to graft failure, development of GVHD or death from any cause. EFS and overall survival (OS) via time to end point analysis was computed using the Kaplan-Meir test.
Results:
A total of 18 adults patients were eligible and further analysed. Median age was 23.5 (14 - 39) years with 14 males and 4 females. All patients were on HU prior to SCT. Median ECOG performance status was 1 (0-1). Indications for transplant were recurrent vaso-occlusive crisis (VOC) refractory to standard therapy in 61%, stroke or other central nervous system (CNS) complication in 38%, recurrent avascular necrosis of joints in 38%, recurrent acute chest syndrome in 22% and severe disease requiring recurrent intensive care unit admission (ICU) in 17%. All donors were related siblings fully matched at 10/10 human leucocyte antigen (HLA) loci with median age of 23.6 years. A total of 11 (61%) of donors were sickle cell carriers with average Hgb S 33%. Stem cell mobilization was with 5 ug/kg GCSF in all donors and the average stem cell collected 11.6 x 106/kg. No significant complications resulted from GCSF use in donors with sickle cell trait.
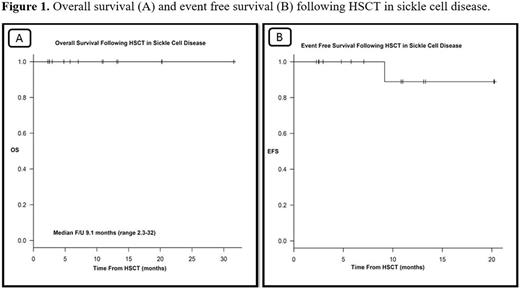
17 patients were conditioned with alemtuzumab / TBI regimen and GVHD prophylaxis with sirolmus. One patient who failed prior transplant received fludarabine, busulfan and thymoglobulin with GVHD prophylaxis as cyclosporine and methotrexate. Median duration of neutrophil engraftment was 20 days and platelet engraftment 2.6 days with half of patient's platelet nadir > 20. Average hemoglobin (Hb) pre- and post-SCT was 83 g/L and 142 g/L respectively. Average hemoglobin (Hb) S pre- and post-SCT was 77% and 13% respectively. Peri-SCT adverse events were 9 (50%) patients experienced neutropenic fever, 12 (67%) mucositis, 6 (33%) diarrhea. None of the patients developed acute or chronic GVHD. No SCD crises developed post SCT. One patient developed aplastic anemia while retaining 71% donor cell chimerism and underwent a second successful SCT from the same donor with fludarabine, cyclophosphamide and thymoglobulin. All patients have stable mixed chimerism resulting in full donor myeloid engraftment and normalization of blood count. With a median follow up of 9.1 months (2.3-32), all patients are alive and free of sickle cell crises. At last follow up, OS and EFS were 100% and 88.9%, respectively as shown in Figure 1.
Conclusion:
Herein, we report that SCT in adults with severe SCD using NMA regimen is safe, feasible and results in amelioration of disease severity. These excellent results are encouraging given the high prevalence and debilitating nature of SCD in our region. Follow up is on-going to assess the feasibility of sirolimus tapering and sustainability of donor chimerism status over time.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.